This page was last modified on:

Iron Transport and Cellular Uptake
Iron kinetics
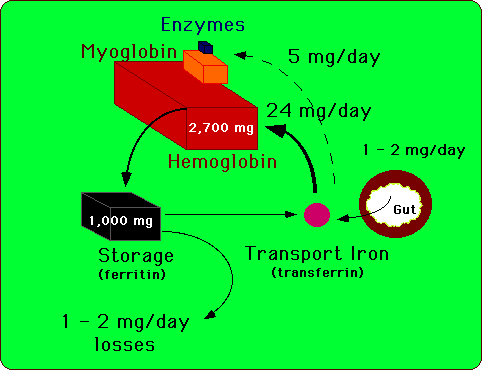 |
| Figure 1. Iron is assiduously conserved and recycled for use
in heme and non-heme enzymes. About 1 to 2 mg of iron are lost each day
to sloughing of skin and mucosal cells of the gastrointestinal and genitouretal
tracts. This obligate iron loss is balanced by iron absorption from the
gastrointestinal tract. Only a small fraction of the 4 grams of body iron
circulate as part of transferrin at any given time. Body iron is most prominently
represented in hemoglobin and in ferritin. |
Only a small proportion of total body iron daily enters or leaves the body's
stores on a daily basis (Figure1). Consequently, intercellular iron transport,
as a part of the iron reutilization process, is quantitatively more important
that intestinal absorption. The greatest mass of iron is found in erythroid
cells, which contain about 80% of the total body endowment. The reticuloendothelial
system recycles a substantial amount of iron from effete red cells, approximating
the amount used by the erythron for new hemoglobin
production.
Transferrin
Of the approximate 3 grams of body iron in the adult male, approximately
3mg or 0.1% circulates in the plasma as an exchangeable pool (Table 1).
Essentially all circulating plasma iron normally is bound to transferrin.
This chelation serves three purposes: it renders iron soluble under physiologic
conditions, it prevents iron-mediated free radical toxicity, and it facilitates
transport into cells. Transferrin is the most important physiological source
of iron for red cells (Ponka, 1997). The liver synthesizes transferrin
and secretes it into the plasma. Transferrins are produced locally in the
testes and CNS. These two sites are relatively inaccessible to proteins
in the general circulation (blood:testis barrier, blood:brain barrier).
The locally synthesized transferrin could play a role in iron metabolism
in these tissues. Information on the function of transferrin produced in
these localized sites is sparce, however.
Plasma transferrin is an 80 kDa glycoprotein with homologous N-terminal
and C-terminal iron-binding domains (reviewed in Huebers and Finch, 1987].
The molecule is related to several other proteins, including ovotransferrin
in bird and reptile eggs (Williams et al., 1982), lactoferrin in extracellular
secretions and neutrophil granules (Mazurier et al., 1983); (Metz-Boutigue
et al., 1984) and melanotransferrin (p97), a protein produced by melanoma
cells (Brown et al., 1982). Ovotransferrin may help protect the developing
embryo in the semi-permeable egg by sequestering iron
that microbes need to grow. Lactoferrin, in secretions such as milk
and tears, might have a similar function. One recent report indicates that
lactoferrin can act as a site-specific DNA binding protein, and could mediate
transcriptional activation. Such a function is, however, at odds with its
existence as an extracellular protein (He and Furmanski, 1995).
X-ray crystal structures exist for human lactoferrin and rabbit
transferrin (reviewed by [Baker and Lindley, 1992]. All members of the
transferrin protein superfamily have similar polypeptide folding patterns.
N-terminal and C-terminal domains are globular moieties of about 330 amino
acids; each of these is divided into two sub-domains, with the iron- and
anion-binding sites in the intersubdomain cleft. The binding cleft opens
with iron release, and closes with iron binding. N- and C-terminal binding
sites are highly similar.
Iron binding by Transferrin
The precise mechanics of iron loading onto transferrin as it leaves
intestinal
epithelial cells or reticuloendothelial cells is unknown. The copper-dependent
ferroxidase, ceruloplasmin, may play a role. Compelling evidence indicates
that the protein is involved in mobilizing tissue iron stores to produce
diferric transferrin (Osaki and Johnson, 1969); (Osaki et al., 1971); (Yoshida
et al., 1995); (Harris et al., 1995).
Transferrin binds iron avidly with a dissociation constant of approximately
1022 M-1 (Aisen and Listowsky, 1980). Ferric iron
couples to transferrin only in the company of an anion (usually carbonate)
that serves as a bridging ligand between metal and protein, excluding water
from two coordination sites (Aisen and Listowsky, 1980); (Harris and Aisen,
1989); (Shongwe et al., 1992). Without the anion cofactor, iron binding
to transferrin is negligible. With it, ferric transferrin is resistant
to all but the most potent chelators. The remaining four coordination sites
are provided by the transferrin protein - a histidine nitrogen, an aspartic
acid carboxylate oxygen, and two tyrosine phenolate oxygens (Bailey et
al., 1988); (Anderson et al., 1989). Available evidence suggests that anion-binding
takes place prior to iron-binding. Iron release from transferrin involves
protonation of the carbonate anion, loosening the metal-protein bond.
Table 1. Distribution and Kinetics of Body Iron
|
Compartment
|
Iron (grams)
|
Percent of Total
|
| Hemoglobin |
2.7
|
66
|
| Myoglobin |
0.2
|
3
|
| Heme Enzymes |
0.008
|
0.1
|
| Non-heme Enzymes |
< 0.0001
|
---
|
Intracellular Storage
(Ferritin) |
1.0
|
30
|
Intracellular Labile Iron
(Chelatable Iron) |
0.07 (?)
|
1
|
Intercellular Transport
(Transferrin) |
0.003
|
0.1
|
Transferrin/Iron Physiology
The sum of all iron binding sites on transferrin constitutes the
total iron binding capacity (TIBC) of plasma. Under normal circumstances,
about one-third of transferrin iron-binding pockets are filled. Consequently,
with the exception of iron overload where all the transferrin binding sites
are occupied, non-transferrin-bound iron in the circulation is virtually
nonexistent. Distribution of plasma and tissue iron can be traced using
59Fe
as a radioactive tag. The subject receives autologous transferrin loaded
with radioactive iron that then can be monitored. Blood samples can be
analyzed at timed intervals to determine the rate of loss of the radioactive
label. Such ferrokinetic studies indicate that the normal half-life of
iron in the circulation is about 75 minutes (Huff et al., 1950). The absolute
amount of iron released from transferrin per unit time is the plasma iron
turnover (PIT).
Such radioactive tracer studies indicate that at least eighty
percent of the iron bound to circulating transferrin is delivered to the
bone marrow and incorporated into newly formed erythrocytes (Jandl and
Katz, 1963); (Finch et al., 1982); Fig. 1). Other major sites of iron delivery
include the liver, which is a primary depot for stored iron, and the spleen.
Hepatic iron is found in both reticuloendothelial cells and hepatocytes.
Reticuloendothelial cells acquire iron primarily by phagocytosis and breakdown
of aging red cells These cells extract the iron from heme and return it
to the circulation bound to transferrin. Hepatocytes take up iron by at
least two different pathways. The first involves receptor-mediated endocytosis
of transferrin. In addition, hepatocytes can take up ionic iron by a process
independent of transferrin (Inman and Wesling-Resnick, 1993).
Ferrokinetics and the Bone Marrow
Given the preeminent role of the bone marrow in the clearance of
labeled iron from the circulation, ferrokinetics provide a window on erythropoietic
activity. Conditions that augment erythrocyte production increase the PIT.
For example, hemolytic anemias such as hereditary spherocytosis and sickle
cell disease induce rapid delivery of transferrin-bound iron to the
marrow. In contrast, disorders that reduce red cell production prolong
the PIT. This picture is seen, for example, with anemia due to Diamond
Blackfan anemia.
When erythrocytes are produced and released into the circulation in
a normal fashion, the process of erythropoiesis is termed "effective".
In patients with certain hemolytic anemias, however, the nascent red cells
are so abnormal they are destroyed before leaving the marrow cavity. In
this circumstance, the erythropoiesis is "ineffective", meaning simply
that the erythropoietic precursors have failed to accomplish their primary
task: the delivery of intact erythrocytes to the circulation. The ferrokinetic
profiles such cases show rapid removal of iron from transferrin with a
delayed entry of label into the pool of circulating red cell hemoglobin.
ß+-thalassemia
is an important example of this pattern of hemolytic anemia with ineffective
erythropoiesis. In ß+-thalassemia, ineffective erythropoiesis
is coupled with a markedly enhanced PIT.
Cellular Iron Uptake
Although transferrin was characterized fifty years ago (Laurell and
Ingelman, 1947), its receptor eluded investigators until the early 1980s.
In a quest to better understand the behavior of neoplastic cells, investigators
prepared monoclonal antibodies against tumor cells. The target of these
monoclonal antibodies later was found to be the cell surface transferrin
receptor glycoprotein (Sutherland et al., 1980; Seligman et al.,
1980).
A broad body of literature now supports the concept that the iron-transferrin
complex is internalized by receptor-mediated endocytosis. The general structure
of the transferrin receptor is shown in Figure 2. This disulfide-linked
homodimer has subunits containing 760 amino acids each (Kuhn et al., 1984);
(Schneider et al., 1983); (Jing and Trowbridge, 1987). Oligosaccharides
account for about 5% of the 90 kDa subunit molecular mass (Reckhow and
Enns, 1988). Four glycosylation sites (three N-linked and one O-linked)
line the protein (Hayes et al., 1992). Glycosylation-defective mutants
have fewer disulfide bridges, bind transferrin less efficiently and are
expressed less prominently on the surface expression than are normal receptors
(Williams and Enns, 1993a); (Williams and Enns, 1993b).
Schematic representation of the transferrin receptor
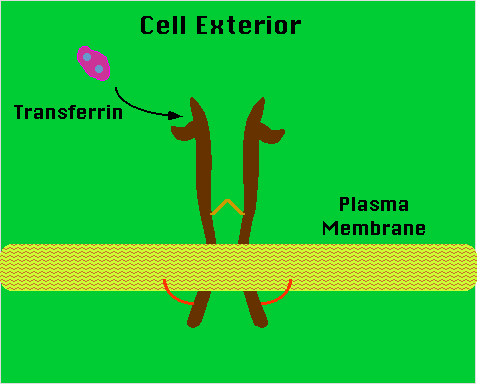 |
| Figure 2. The molecule is a transmembrane homodimer linked by
disulfide bonds. An acyl group attached to the cytoplasmic tail of the
molecule anchors the assembly to the plasma membrane. |
The transmembrane domain, between amino acids 62 and 89, functions
as an internal signal peptide, as none exits at the N-terminal end (Zerial
et al., 1986). A molecule of fatty acid (usually palmitate) covalently
links each subunit to the internal edge of the transmembrane domain and
could play a role in membrane localization. Interestingly, non-acylated
mutants mediate faster iron uptake than normal receptors (Alvarez et al.,
1990); (Jing and Trowbridge, 1990). The transferrin binding regions of
the protein are unidentified (Williams and Enns, 1993a); (Williams and
Enns, 1993b). Efforts to crystallize transferrin receptor protein are underway.
Iron is taken into cells by receptor-mediated endocytosis of monoferric
and diferric transferrin (Karin and Mintz, 1981); (Klausner et al., 1983);
(Iacopetta and Morgan, 1983); (Fig. 3). Receptors on the outer face of
the plasma membrane bind iron-loaded transferrin with a very high affinity.
The C-terminal domain of transferrin appears to mediate receptor binding
(Zak et al., 1994). Diferric transferrin binds with higher affinity than
monoferric transferrin or apotransferrin (Huebers et al., 1984); (Young
et al., 1984). The dissociation constant (Kd) for bound diferric transferrin
ranges from 10-7 M to 10-9 M at physiologic pH, depending
on the species and tissue assayed (Stein and Sussman, 1983); (Sawyer and
Krantz, 1986). The Kd of monoferric transferrin is approximately 10-6
M. The concentration of circulating transferrin is about 25 mM.
Therefore, cellular transferrin receptors ordinarily are fully saturated.
After binding to its receptor on the cell surface, transferrin
is rapidly internalized by invagination of clathrin-coated pits with formation
of endocytic vesicles (Figure 3). This process requires the short, 61 amino
acid intracellular tail of the transferrin receptor molecule (Rothenberger
et al., 1987); (Alvarez et al., 1990); (McGraw and Maxfield, 1990); (Girones
et al., 1991); (Miller et al., 1991). Receptors with truncated N-terminal
cytoplasmic domains do not recycle (Rothenberger et al., 1987). This portion
of the molecule contains a conserved tyrosine-threonine-arginine-phenylalanine
(YTRF) sequence which functions as a signal for endocytotic internalization
(Collawn et al., 1993). Genetically engineered addition of a second YTRF
sequence enhances receptor endocytosis (Collawn et al., 1993). A number
of stimuli reversibly phosphorylate the serine residue adjacent to the
YTRF sequence, at position 24 by the action of protein kinase C (Davis
et al., 1986). The role of receptor phosphorylation is unclear. Despite
removal of the phosphorylation site by site-directed mutagenesis, the transferrin
receptor recycles normally (Rothenberger et al., 1987).
Receptor-mediated transferrin endocytosis
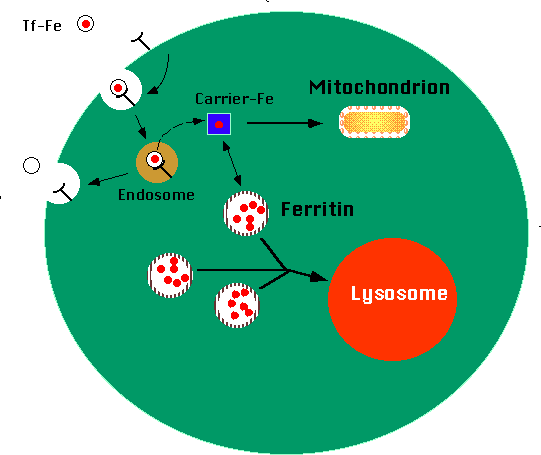 |
| Figure 3. Ferro-transferrin binds to transferrin receptors on
the external surface of the cell. The complex is internalized into an endosome,
where the pH is lowered to about 5.5. Iron separates from the transferrin
molecule, moving into the cell cytoplasm. Here, an iron transport molecule
shuttles the iron to various points in the cell, including mitochondria
and ferritin. Ferritin molecules accumulate excess iron. Lysosomes engulf
aggregates of ferritin molecules in a process termed "autophagy". |
An ATP-dependent proton pump lowers the pH of the endosome to
about 5.5 (Van Renswoude et al., 1982); (Dautry-Varsat et al., 1983); (Paterson
et al., 1984); (Yamashiro et al., 1984). The acidification of the endosome
weakens the association between iron and transferrin. Even at pH 5.5, Fe3+
would not normally dissociate from transferrin in the several minutes between
its endocytosis and the return of transferrin apoprotein to the cell surface
(Ciechanover et al., 1983). A plasma membrane oxidoreductase reduces transferrin
bound iron from the Fe3+ state to Fe2+, directly
or indirectly facilitating the removal of iron from the protein (Low et
al., 1987); (Thorstensen and Romslo, 1988); (Nunez et al., 1990). Conformational
changes in the transferrin receptor also play a role in iron release (Bali
et al., 1991); (Sipe and Murphy, 1991).
Rather than entering lysosomes for degradation, as do ligands
in other receptor-mediated endocytosis pathways, intact receptor-bound
apotransferrin recycles to the cell surface, where neutral pH promotes
detachment into the circulation (Zak and Aisen, 1990). Thus the preservation
and re-use of transferrin are accomplished by pH-dependent changes in the
affinity of transferrin for its receptor (Van Renswoude et al., 1982);
(Klausner et al., 1983); (Dautry-Varsat et al., 1983). Exported apotransferrin
binds additional iron and undergoes further rounds of iron delivery to
cells. The average transferrin molecule, with a half-life of eight days,
may be used up to one hundred times for iron delivery (Harford et al.,
1994).
Topologically, the cell exterior and the endosome interior are
equivalent compartments. The primary role of the transferrin-transferrin
receptor interaction is to bring iron into the vicinity of the cell surface,
thereby increasing the likelihood of iron uptake. Following its release
from transferrin within the endosome, iron must traverse the plasma membrane
to enter the cytosol proper. The molecules effecting this transport have
not been identified, but the process may be carrier-mediated (Egyed, 1988).
Two anemic, mutant animals, the Belgrade rat (b/b) and the hemoglobin deficit
mouse (hbd/hbd) appear to have lesions at or near this step. Their cells
take up ferrotransferrin into endosomes, but fail to release iron into
the cytoplasm (Garrick et al., 1987); (Garrick et al., 1993). The molecular
basis of the defects in these animals have not been elucidated.
The endosomal transporter may reside on the plasma membrane of
the cell prior to endocytosis (Pollack, 1992). If so, it should be oriented
to transport iron directly into the cell, without the assistance of transferrin.
Such non-transferrin-bound iron uptake activities have been characterized
in tissue culture. This uptake system could function constitutively but
inefficiently. Coupling the transferrin cycle to transport across the plasma
membrane might augment iron uptake by creating an iron-rich environment
for the transporter within the endosome. This same elusive transport molecule
could also be involved in intestinal iron uptake. The phenotype of the
mk/mk mouse (see above) suggests that red cell iron uptake and intestinal
iron uptake share a common component which could be the 'endosomal' transporter.
Once inside the cell cytoplasm, iron appears to be bound by a
low molecular weight carrier molecule, which may assist in delivery to
various intracellular locations including mitochondria (for heme biosynthesis)
and ferritin (for storage). The identity of the intracellular iron carrier
molecule(s) remains unknown. The amount of iron in transit within the cell
at any given time is minuscule and defies precise measurement. This minute
pool of transit iron, which is believed to be in the Fe2+ oxidation
state, is the biologically active form of the element. Metabolically inactive
iron, stored in ferritin and hemosiderin, is in equilibrium with exchangeable
iron bound to the low molecular weight carrier molecule (Figure 3).
Both prokaryotes and eukaryotes produce ferritin molecules for
iron storage. Ferritins are complex twenty-four subunit heteropolymers
of H (for heavy or heart) and L (for light or liver) protein subunits (Theil,
1987). L subunits are 19.7 kDa in mass, with isoelectric points of 4.5-5.0;
H subunits are 21 kDa with isoelectric points of 5.0-5.7. The subunits
of the ferritin molecule form a sphere with a central cavity in which up
to 4500 atoms of crystalline iron is stored in the form of poly-iron-phosphate
oxide (Theil, 1987). Eight channels through the sphere are lined by hydrophilic
amino acid residues (along the three-fold axes of symmetry) and six more
are lined by hydrophobic residues (along the four-fold axes; [Harrison
et al., 1986].) Strong interspecies amino acid conservation exists in the
residues that line the hydrophilic channels, while marked variation exists
in those along the hydrophobic passages. Hydrophilic channels terminate
with aspartic acid and glutamic acid residues , and are lined by serine,
histidine and cysteine residues (all of which potentially bind metal ligands).
The evolutionary conservation of the hydrophilic channels suggests that
they provide the route for iron entry and exit from the ferritin shell,
but this contention remains unproved. Little is known about how iron is
released from ferritin for use.
Although the two ferritin chains are highly homologous, only H
ferritin has ferroxidase activity. A mechanism involving dioxygen converts
ferrous to ferric iron, promoting incorporation into ferritin (Levi et
al., 1988); (Lawson et al., 1991). The composition of ferritin shells varies
from H-subunit homopolymers to L-subunit homopolymers, and includes all
possible combinations between the two. Isoelectric focusing of ferritin
from a particular tissue reveals multiple bands representing shells with
different subunit compositions. These isoferritins, as they are called,
show tissue specific variation (Drysdale, 1988). Ferritin from liver, for
instance, is rich in L-subunits, as is that from the spleen. In contrast,
the heart has ferritin rich in H-subunits. Increased H subunit content
correlates with increased iron utilization, while increased L subunit content
correlates with increased iron storage (Drysdale, 1988); (Theil, 1987).
The H:L ratio rises with activation of heme synthesis or cell proliferation
(Pattanapanyasat et al., 1987); (McClarty et al., 1990). Ferritin thus
provides a flexible reserve of iron.
Ferritin molecules aggregate over time to form clusters, which
are engulfed by lysosomes and degraded (Iancu et al., 1977); (Bridges,
1987); Figure 3). The end-product of this process, hemosiderin, is an amorphous
agglomerate of denatured protein and lipid interspersed with iron oxide
molecules (reviewed by (Wixom et al., 1980). In cells overloaded with iron,
lysosomes accumulate large amounts of hemosiderin which can be visualized
by Prussian blue staining. Although the iron enmeshed in this insoluble
compound constitutes an endstage product of cellular iron storage, it remains
in equilibrium with soluble ferritin. Ferritin iron, in turn, is in equilibrium
with iron complexed to low molecular weight carrier molecules. Therefore
the introduction into the cell of an effective chelator captures iron from
the low molecular weight "toxic iron" pool, draws iron out of ferritin,
and eventually depletes iron from hemosiderin as well, though only very
slowly. As might be expected, the bioavailability of hemosiderin iron is
much lower than that of iron stored in ferritin.
Non-Transferrin-Bound Iron Uptake
Alhough compelling evidence exists that the transferrin cycle is
important for iron acquisition by the erythron (Ponka and Schulman, 1993;
Ponka, 1997)), other tissues can import iron by alternative mechanisms.
Some patients and mutant mice that have little or no circulating transferrin
(Heilmeyer, 1966); (Goya et al., 1972); (Bernstein, 1987); (Huggenvik et
al., 1989). Despite severe hypochromic, microcytic anemia, non-erythroid
tissues are grossly normal. While the red cells suffer from iron deficiency,
serum iron levels (iron not bound to transferrin) are elevated, and excess
iron is deposited in the liver. The iron-deprived bone marrow likely signals
the gut to increase absorption, exacerbating tissue iron excess. Ponka
and Schulman speculate that non-erythroid cells depend less on transferrin
because their modest iron needs can be met by turnover of endogenous ferritin
and heme iron. Red cells are more vulnerable because of greater iron use
to form hemoglobin (Ponka and Schulman, 1993; Ponka, 1997). The transferrin
cycle could serve primarily to enhance iron uptake by tissues with a great
demand for the element.
Iron overload produces fully saturated transferrin and non-transferrin
bound iron circulating in a chelatable, low molecular weight form (Hershko
et al., 1978); (Hershko and Peto, 1978); (Craven et al., 1987); (Grootveld
et al., 1989). This iron is weakly complexed to albumin, citrate, amino
acids and sugars, and behaves differently from iron associated with transferrin.
Non-hematopoietic tissues, particularly the liver, endocrine organs, kidneys
and heart preferentially take up this iron.
Radiolabeled iron administered to mice with and without available
transferrin binding capacity has quite different patterns of distribution
(Craven et al., 1987). In normal animals, hematopoietic tissues are the
prime sites of uptake. When free transferrin sites are absent, however,
most iron is deposited in the liver and pancreas, indicating that these
organs serve as iron reservoirs in the situation of iron overload. Notably,
this pattern of distribution is similar to that seen in idiopathic hemochromatosis.
These data support the idea that, while the transferrin pathway is important
for meeting the needs of the erythron, it is not essential for iron uptake
by all tissues.
Kaplan and coworkers have studied iron incorporation from FeNH4
citrate (Sturrock et al., 1990); (Kaplan et al., 1991). Intriguingly, they
find that transferrin-independent uptake increases in direct proportion
to the concentration of this compound, similar to hepatic uptake of non-transferrin-bound
iron in patients with saturated transferrin. They speculate that this is
a protective alternative pathway that removes the toxic metal from the
circulation. Other investigators have described similar uptake in HepG2
cells, and shown that it is reversible by addition of chelating compounds
(Randell et al., 1994).
A non-transferrin iron uptake mechanism with different properties
has been described in K562 erythroleukemia cells (Inman and Wessling-Resnick,
1993). In the absence of ferric transferrin, iron uptake into K562 cells
is sensitive to treatment with trypsin, suggesting that it requires a protein
carrier. Higher ambient iron concentrations do not increase cellular iron
uptake. As discussed above, this transport may be accomplished by the same
machinery responsible for passage of iron out of transferrin cycle endosomes
into the cytoplasm (Pollack, 1992). These two processes accomplish essentially
the same task. The putative endosomal iron transporter must be oriented
to transport iron from an endocytosed extracellular compartment into the
cytoplasm. This transporter may exist on the cell surface prior to receptor-mediated
endocytosis, with the capacity to transport iron to a modest extent. This
activity is not restricted to erythroid cells. PHA-stimulated human peripheral
lymphocytes have a similar transferrin-independent iron uptake mechanism
(Hamazaki and Glass, 1992).
References:
-
Aisen, P., and Listowsky, I. (1980). Iron transport and storage proteins.
Annual Reviews of Biochemistry 49, 357-93.
-
Alvarez, E., Girones, N., and Davis, R. J. (1990). Inhibition of receptor-mediated
endocytosis of diferrin transferrin is associated with covalent modification
of the transferrin receptor with palmitic acid. Journal of Biological Chemistry
265, 16644-55.
-
Anderson BF, Baker HM, Norris GE, Rice DW, and Baker EN. (1989). Structure
of human lactoferrin: Crystallographic structure analysis and refinement
at 2.8 A resolution. J. Mol. Biol. 209, 711.
-
Bailey S,Evans RW, Garatt RC, et al. (1988). Molecular structure of serum
transferrin at 3.3-A resolution. Biochem. 27, 5804.
-
Bali, P. K., Zak, O., and Aisen, P. (1991). A new role for the transferrin
receptor in the release of iron from transferrin. Biochemistry 30, 324-8.
-
Baker, E. N., and Lindley, P. F. (1992). New perspectives on the structure
and function of transferrins. Journal of Inorganic Biochemistry 47, 147-160.
-
Bernstein, S. E. (1987). Hereditary hypotransferrinemia with hemosiderosis,
a murine disorder resembling human atransferrinemia. Journal of Laboratory
and Clinical Medicine 110, 690-705.
-
Bridges, K. R. (1987). Ascorbic acid inhibits lysosomal autophagy of ferritin.
J. Biol. Chem. 262, 14773.
-
Brown, J. P., Henwick, R. M., and al., e. (1982). Human melanoma-associated
antigen p97 is structurally and functionally related to transferrin. Nature
296, 171.
-
Ciechanover, A., Schwartz, A. L., Dautry-Varsat, A., and Lodish, H. F.
(1983). Kinetics of internalization and recycling of transferrin and the
transferrin receptor in a human hepatoma cell line. Journal of Biological
Chemistry 258, 9681-9.
-
Collawn, J. F., Lai, A., Domingo, D., Fitch, M., Hatton, S., and Trowbridge,
I. S. (1993). YTRF is the conserved internalization signal of the transferrin
receptor, and a second YTRF signal at position 30-34 enhances endocytosis.
Journal of Biological Chemistry 268, 21686-92.
-
Craven, C. M., Alexander, J., Eldridge, M., Kushner, J. P., Bernstein,
S., and Kaplan, J. (1987). Tissue distribution and clearance kinetics of
non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model
for hemochromatosis. Proceedings of the National Academy of Sciences (USA)
84, 3457.
-
Davis, R. J., Johnson, G. L., Kelleher, D. J., Anderson, J. K., Mole, J.
E., and Czech, M. P. (1986). Identification of serine 24 as the unique
site on the transferrin receptor phosphorylated by protein kinase C. Journal
of Biological Chemistry 261, 9034.
-
Dautry-Varsat, A., Ciechanover, A., and Lodish, H. F. (1983). pH and the
recycling of transferrin during receptor-mediated endocytosis. Proceedings
of the National Academy of Sciences (USA) 80, 2258-62.
-
Drysdale, J. W. (1988). Human ferritin gene expression [Review]. Prog.
Nucleic Acid Res. 35, 127.
-
Egyed, A. (1988). Carrier mediated iron transport through erythroid cell
membrane. British Journal of Haematology 68, 483-6.
-
Finch C, Huebers H, Eng M,Miller L. (1982). Effect of transfused reticulocytes
on iron exchange. Blood 59, 364-9.
-
Garrick, L. M., Edwards, J. A., Hoke, J. E., and Bannerman, R. M. (1987).
Diminished acquisition of iron by reticulocytes from mice with hemoglobin
deficit. Experimental Hematology 15, 671-5.
-
Garrick, M. D., Gniecko, K., Liu, Y., Cohan, D. S., and Garrick, L. M.
(1993). Transferrin and the transferrin cycle in Belgrade rat reticulocytes.
Journal of Biological Chemistry 20, 14867-14874.
-
Girones, N., Alvarez, E., Seth, A., Lin, I. M., Latour, D. A., and Davis,
R. J. (1991). Mutational analysis of the cytoplasmic tail of the human
transferrin receptor. Identification of a sub-domain that is required for
rapid endocytosis. Journal of Biological Chemistry 266, 19006-12.
-
Goya, N., Miyazaki, S., Kodate, S., and Ushio, B. (1972). A family of congenital
atransferrinemia. Blood 40, 239 - 245.
-
Grootveld, M., Bell, J. D., Halliwell, B., Aruoma, O. I., Bomford, A.,
and Sadler, P. J. (1989). Non-transferrin-bound iron in plasma or serum
from patients with idiopathic hemochromatosis. Characterization by high
performance liquid chromatography and nuclear magnetic resonance spectroscopy.
J. Biol. Chem. 264, 4417.
-
Harford, J. B., Rouault, T. A., Huebers, H. A., and Klausner, R. D. (1994).
Molecular mechanisms of iron metabolism. In The Molecular Basis of Blood
Diseases, G. Stamatoyannopoulos, A. W. Nienhuis, P. W. Majerus and H. Varmus,
eds. (Philadelphia: W.B. Saunders Co.), pp. 351-378.
-
Hamazaki, S., and Glass, J. (1992). Non-transferrin dependent 59Fe uptake
in phytohemagglutinin-stimulated human peripheral lymphocytes. Experimental
Hematology 20, 436-41.
-
Harris, D. C., and Aisen, P. (1989). Physical biochemistry of the transferrins.
In Iron Carriers and Iron Proteins, T. M. Loehr, H. B. Gray and A. B. P.
Lever, eds. (Weinheim: VCH Publishers), pp. 239-351.
-
Harris, Z. L., Takahashi, Y., Miyajima, H., Serizawa, M., MacGillivray,
R. T. A., and Gitlin, J. D. (1995). Aceruloplasminemia: Molecular characterization
of this disorder of iron metabolism. Proceedings of the National Academy
of Sciences (USA) 92, 2539-2543.
-
Harrison, P. M., Treffry, A., and Lilley, T. H. (1986). Ferritin as an
iron storage protein: mechanisms of iron uptake. J. Inorg. Biochem. 27,
287.
-
Hayes, G. R., Enns, C. A., and Lucas, J. J. (1992). Identification of the
O-linked glycosylation site of the human transferrin receptor. Glycobiology
2, 355.
-
He J and Furmanski P. (1995). Sequence specificity and transcriptional
activation in the binding of lactoferrin to DNA. Nature 373, 721-724.
-
Heilmeyer, L. (1966). Atransferrinemias [German]. Acta Haematologica 36,
40.
-
Hershko, C., Graham, G., Bates, G. W., and Rachmilewitz, E. A. (1978).
Non-specific serum iron in thalassemia: an abnormal serum iron fraction
of potential toxicity. Br. J. Haematol. 40, 255.
-
Hershko, C., and Peto, T. E. (1987). Non-transferrin plasma iron [editorial].
Br. J. Haematol. 66, 149.
-
Huebers, H. A., Huebers, E., Csiba, E., and Finch, C. A. (1984). Heterogeneity
of the plasma iron pool: explanation of the Fletcher-Huehns phenomenon.
Am. J. Physiol. 247, R280.
-
Huebers HA and Finch CA. (1987). The physiology of transferrin and transferrin
receptors. Physiological Reviews 67, 520.
-
Huff, R. L., Hennessey, T. G., Austin, R. E., Garcia, J. F., Roberts, B.
M., and Lawrence, J. H. (1950). Plasma and red cell iron turnover in normal
subjects and in patients having various hematopoietic disorders. Journal
of Clinical Investigation 29, 1041.
-
Huggenvik, J. I., Craven, C. M., Idzerda, R. L., Bernstein, S., Kaplan,
J., and McKnight, G. S. (1989). A splicing defect in the mouse transferrin
gene leads to congenital atransferrinemia. Blood 74, 482-6.
-
Iacopetta, B. J., and Morgan, E. H. (1983). The kinetics of transferrin
endocytosis and iron uptake from transferrin in rabbit reticulocytes. J.
Biol. Chem. 258, 9108.
-
Iancu, T. C., Neustein, H. B., and Landing, B. H. (1977). The liver in
thalassemia mahor: ultrastructural observations. In Ciba Foundation Symposium
(New York: Elsevier), pp. 293-316.
-
Inman RS and Wessling-Resnick M. (1993). Characterization of transferrin-independent
iron transport in K562 cells. Unique properties provide evidence for multiple
pathways of iron uptake. J Biol Chem 268: 8521-8528.
-
Jandl JH and Katz JH. (1963). The plasma-to-cell cycle of transferrin.
Journal of Clinical Investigation 42, 314.
-
Jing, S. Q., and Trowbridge, I. S. (1987). Identification of the intermolecular
disulfide bonds of the human transferrin receptor and its lipid-attachment
site. EMBO Journal 6, 327-31.
-
Jing, S. Q., and Trowbridge, I. S. (1990). Nonacylated human transferrin
receptors are rapidly internalized and mediate iron uptake. Journal of
Biological Chemistry 265, 11555-9.
-
Kaplan, J., Jordan, I., and Sturrock, A. (1991). Regulation of the transferrin-independent
iron transport system in cultured cells. Journal of Biological Chemistry
266, 2997-3004.
-
Karin, M., and Mintz, B. (1981). Receptor-mediated endocytosis of transferrin
in developmentally totipotent mouse teratocarcinoma stem cells. J. Biol.
Chem. 256, 3245.
-
Klausner, R. D., van Renswoude, J., Ashwell, G., Kempf, C., Schechter,
A. M., Dean, A., and Bridges, K. R. (1983). Receptor-mediated endocytosis
of transferrin in K562 cells. Journal of Biological Chemistry 258, 4715-24.
-
Kuhn, L. C., McClelland, A., and Ruddle, F. H. (1984). Gene transfer, expression
and molecular cloning of the human transferrin receptor gene. Cell 37,
95-103.
-
Lawson, D. M., Artymiuk, p. J., Yewdall, S. J., Livingstone, J. C., Treffry,
A., Luzzago, A., Levi, S., Arosio, P., Cesareni, G., Thomas, C. D., Shaw,
W., and Harrison, P. M. (1991). Solving the structure of human H ferritin
by genetically engineering intermolecular crystal contacts. Nature 349,
541.
-
Levi, S., Luzzago, A., Cesareni, G., Cozzi, A., Franceschinelli, F., Albertini,
A., and Arosio, P. (1988). Mechanism of ferritin iron uptake: activity
of the H-chain and deletion mapping of the ferro-oxidase site. A study
of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain
ferritins, and two H-chain deletion mutants. J. Biol. Chem. 263, 18086.
-
Low, H., Grebing, C., Lindgren, A., Tally, M., Sun, I. L., and Crane, F.
L. (1987). Involvement of transferrin in the reduction of iron by the transplasma
membrane electron transport system. J. Bioenerg. Biomembr. 19, 535.
-
Mazurier J, Metz-Boutigue ., et al. (1983). Human lactotransferrin: Molecular,
functional and evolutionary comparisons with human serum transferrin and
hen ovotransferrin. Experientia 39, 135.
-
McClarty, G., Chan, A. K., Choy, B. K., and Wright, J. A. (1990). Increased
ferritin gene expression is associated with increased ribonucleotide reductase
gene expression and the establishment of hydroxyurea resistance in mammalian
cells. J. Biol. Chem. 265, 7539.
-
McGraw, T. E., and Maxfield, F. R. (1990). Human transferrin receptor internalization
is partly dependent upon an aromatic amino acid in the cytoplasmic domain.
Cell Regulation 1, 369-77.
-
Metz-Boutigue MH, Jolies, J., and al., e. (1984). Human lactotransferrin:
Amino acid sequence and structural comparison with other transferrins.
Eur. J. Biochem. 145, 659.
-
Miller, K., Shipman, M., Trowbridge, I. S., and Hopkins, C. R. (1991).
Transferrin receptors promote the formation of clathrin lattices. Cell
65, 621.
-
Nunez, M.-T., Gaete, V., Watkins, J. A., and Glass, J. (1990). Mobilization
of iron from endocytic vesicles. The effects of acidification and reduction.
Journal of Biological Chemistry 265, 6688-92.
-
Osaki, S., and Johnson, D. A. (1969). Mobilization of liver iron by ferroxidase
(ceruloplasmin). J. Biol. Chem. 244, 5757-5765.
-
Osaki, S., Johnson, D. A., and Frieden, E. (1971). The mobilization of
iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase
I. J. Biol. Chem. 246, 3018-3023.
-
Paterson, S., Armstrong, N. J., Iacopetta, B. J., McArdle, H. J., and Morgan,
E. H. (1984). Intravesicular pH and iron uptake by immature erythroid cells.
Journal of Cellular Physiology 120, 225-32.
-
Pattanapanyasat, K., Hoy, T. G., and Jacobs, A. (1987). The response of
intracellular and surface ferritin after T-cell stimulation in vitro. Clin.
Sci. (London) 73, 605.
-
Pollack, S. (1992). Receptor-mediated iron uptake and intracellular iron
transport [review]. American Journal of Hematology 39, 113.
-
Ponka, P., and Schulman, H. M. (1993). Regulation of heme biosynthesis:
distinct features in erythroid cells. Stem Cells 11 (supplement 1), 24-35.
-
Ponka P. 1997. Tissue-specific regulation of iron metabolism and heme synthesis:
distinct control mechanisms in erythroid cells.
-
Randell, E. W., Parkes, J. G., Olivieri, N. F., and Templeton, D. M. (1994).
Uptake of non-transferrin bound iron by both reductive and non-reductive
processes is modulated by intracellular iron. Journal of Biological Chemistry
269, 16046-53.
-
Reckhow, C. L., and Enns, C. A. (1988). Characterization of the transferrin
receptor in tunicamycin-treated A431 cells. Journal of Biological Chemistry
263, 7297.
li> Rothenberger, S., Iacopetta, B. J., and Kuhn, L. C. (1987). Endocytosis
of the transferrin receptor requires the cytoplasmic domain but not its
phosphorylation site. Cell 49, 423-31.
-
Schneider, C., Kurkinen, M., and Greaves, M. (1983). Isolation of cDNA
clones for the human transferrin receptor. EMBO Journal 2, 2259-63.
-
Shongwe, M. S., Smith, C. A., Ainscough, E. W., Baker, H. M., Brodie, A.
M., and Baker, E. N. (1992). Anion binding by human lactoferrin: Results
from crystallographic and physicochemical studies. Biochemistry 31, 4451.
-
Sawyer, S. T., and Krantz, S. B. (1986). Transferrin receptor number, synthesis
and endocytosis during erythropoietin-induced maturation of Friend virus-infected
erythroid cells. Journal of Biological Chemistry 261, 9187.
-
Seligman, P., Schleicher, R., and Allen, R. (1979). Isolation and characterization
of the transferrin receptor from human placenta. :. Journal of Biological
Chemistry 254, 9943.
-
Sipe, D. M., and Murphy, R. F. (1991). Binding to cellular receptors results
in increased iron release from transferrin at mildly acidic pH. Journal
of Biological Chemistry 266, 8002-7.
-
Stein, B. S., and Sussman, H. H. (1983). Peptide mapping of the human transferrin
receptor in normal and transformed cells. J. Biol. Chem. 258, 2668.
-
Sturrock, A., Alexander, J., Lamb, J., Craven, C. M., and Kaplan, J. (1990).
Characterization of a transferrin-independent uptake system for iron in
HeLa cells. Journal of Biological Chemistry 265, 3139-45.
-
Theil, E. C. (1987). Ferritin: structure, gene regulation and cellular
function in animals, plants and microorganisms. Annual Reviews of Biochemistry
56, 289.
-
Thorstensen, K., and Romslo, I. (1988). Uptake of iron from transferrin
by isolated rat hepatocytes. A redox-mediated plasma membrane process?
Journal of Biological Chemistry 263, 8844-50.
-
Van Renswoude, J., Bridges, K. R., Harford, J. B., and Klausner, R. D.
(1982). Receptor-mediated endocytosis and the uptake of iron in K562 cells:
Identification of a non-lysosomal acidic compartment. Proceedings of the
National Academy of Sciences (USA) 79, 6186-90.
-
Williams J, Ellerman TC, et al. (1982). The primary structure of hen ovotransferrin.
Eur. J. Biochem. 122, 297.
-
Williams, A. M., and Enns, C. A. (1993a). A mutated transferrin receptor
lacking asparagine-linked glycosylation sites shows reduced functionality
and an association with binding immunoglobulin protein. Journal of Biological
Chemistry 266, 17648.
-
Williams, A. M., and Enns, C. A. (1993b). A region of the C-terminal portion
of the human transferrin receptor contains and asparagine-linked glycosylation
site critical for receptor structure and function. Journal of Biological
Chemistry 268, 12780.
-
Wixom, R., Prutkin, L., and Munro, H. (1980). Hemosiderin: nature, formation
and significance. International Review of Expermental Pathology 22, 193
- 225.
-
Yamashiro, D. J., Tycko, B., Fluss, S. R., and Maxfield, F. R. (1984).
Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment
in the recycling pathway. Cell 37, 789-800.
-
Young, S. P., Bomford, A., and Williams, R. (1984). The effect of the iron
saturation of transferrin on its binding and uptake by rabbit reticulocytes.
Biochem. J. 219, 505.
-
Yoshida, K., Furihata, K., Takeda, S., Nakamura, A., Yamamoto, K., Morita,
H., Hiyamuta, S., Ikeda, S., Shimizu, N., and Yanagisawa, N. (1995). A
mutation in the ceruloplasmin gene is associated with systemic hemosiderosis
in humans. Nat. Genet. 9, 267-273.
-
Zak, O., Trinder, D., and Aisen, P. (1994). Primary receptor-recognition
site of human transferrin is in the C-terminal lobe. J. Biol. Chem. 269,
7110.
-
Zerial, M., Melancon, P., Schneider, C., and Garoff, H. (1986). The transmembrane
segment of the human transferrin receptor functions as a signal peptide.
EMBO Journal 5, 1543.














