revised April 14, 2002
Hemoglobin Synthesis
Hemoglobin synthesis requires the coordinated production of heme
and globin. Heme is the prosthetic group that mediates reversible binding
of oxygen by hemoglobin. Globin is the protein that surrounds and protects
the heme molecule.
Heme Synthesis
Heme is synthesized in a complex series of steps involving enzymes in the
mitochondrion and in the cytosol of the cell (Figure 1). The first step in heme
synthesis
takes place in the mitochondrion, with the condensation of succinyl CoA
and glycine by ALA synthase to form 5-aminolevulic acid (ALA). This
molecule is transported to the cytosol where a series of reactions produce
a ring structure called coproporphyrinogen III. This molecule returns to
the mitochondrion where an addition reaction produces protoporhyrin IX.
Heme Biosynthesis
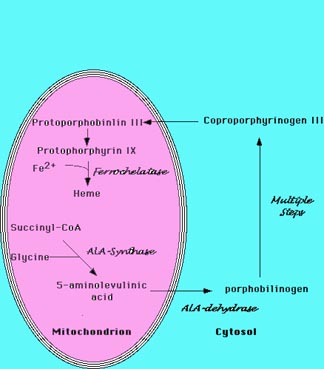 |
Figure 1 Heme Biosynthesis.
The sythesis of heme is a complex process that involves multiple enzymatic
steps.
The process begins in the mitochondrion with the condensation of succinyl-CoA
and
glycine to form 5-aminolevulinic acid. A series of steps in the cytoplasm
produce
coproporphrynogen III, which re-enters the mitochondrion. The final enzymatic
steps
produce heme. |
The enzyme ferrochelatase inserts iron into the ring structure of protoporphyrin
IX to produce heme. Deranged production of heme produces a variety
of anemias. Iron deficiency, the world's most common cause of anemia, impairs
heme synthesis thereby producing anemia. A number of drugs and toxins directly
inhibit heme production by interfering with enzymes involved in heme
biosynthesis.
Lead commonly produces substantial anemia by inhibiting heme synthesis,
particularly in children.
Globin Synthesis
Two distinct globin chains (each with its individual heme molecule)
combine to form hemoglobin. One of the chains is designated alpha. The
second chain is called "non-alpha". With the exception of the very first
weeks of embryogenesis, one of the globin chains is always alpha. A number
of variables influence the nature of the non-alpha chain in the hemoglobin
molecule. The fetus has a distinct non-alpha chain called gamma. After
birth, a different non-alpha globin chain, called beta, pairs with the
alpha chain. The combination of two alpha chains and two non-alpha chains
produces a complete hemoglobin molecule (a total of four chains per molecule).
The combination of two alpha chains and two gamma chains form "fetal"
hemoglobin, termed "hemoglobin F". With the exception of the first 10 to
12 weeks after conception, fetal hemoglobin is the primary hemoglobin in
the developing fetus. The combination of two alpha chains and two beta
chains form "adult" hemoglobin, also called "hemoglobin A". Although hemoglobin
A is called "adult", it becomes the predominate hemoglobin within about 18
to 24 weeks of birth.
The pairing of one alpha chain and one non-alpha chain produces a hemoglobin
dimer (two chains). The hemoglobin dimer does not efficiently deliver oxygen,
however. Two dimers combine to form a hemoglobin tetramer, which is the
functional form of hemoglobin. Complex biophysical characteristics of the
hemoglobin tetramer permit the exquisite control of oxygen uptake in the
lungs and release in the tissues that is necessary to sustain life.
The genes that encode the alpha globin chains are on chromosome 16 (Figure
2).
Those that encode the non-alpha globin chains are on chromosome 11. Multiple
individual genes are expressed at each site. Pseudogenes are also present
at each location. The alpha complex is called the "alpha globin locus",
while the non-alpha complex is called the "beta globin locus". The expression
of the alpha and non-alpha genes is closely balanced by an unknown mechanism.
Balanced gene expression is required for normal red cell function. Disruption
of the balance produces a disorder called thalassemia.
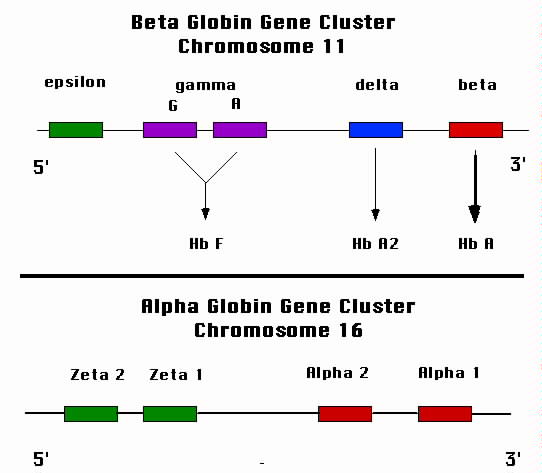 |
| Figure 2. Schematic representation of the globin gene loci. The
lower panel shows the alpha globin locus that resides on chromosome 16. Each of the
four alpha globin genes contribute to the synthesis of the alpha globin protein.
The upper panel shows the beta globin locus. The two gamma globin genes are active
during fetal growth and produce hemoglobin F. The "adult" gene, beta, takes over
after birth. |
Alpha Globin Locus
Each chromosome 16 has two alpha globin genes that are aligned one after
the other on the chromosome. For practical purposes, the two alph globin
genes (termed alpha1 and alpha2) are identical. Since each cell has two
chromosomes 16, a total of four alpha globin genes exist in each cell.
Each of the four genes produces about one-quarter of the alpha globin chains
needed for hemoglobin synthesis. The mechanism of this coordination is
unknown. Promoter elements exist 5' to each alpha globin gene. In addition,
a powerful enhancer region called the locus control region (LCR) is required
for optimal gene expression. The LCR is many kilobases upstream of the
alpha globin locus. The mechanism by which DNA elements so distant from
the genes control their expression is the source of intense investigation.
The transiently expressed embryonic genes that substitute for alpha very
early in development, designated zeta, are also in the alpha globin locus.
Beta Globin Locus
The genes in the beta globin locus are arranged sequentially from 5' to
3' beginning with the gene expressed in embryonic development (the first
12 weeks after conception; called episolon). The beta globin locus ends
with the adult beta globin gene. The sequence of the genes is: epsilon,
gamma, delta, and beta. There are two copies of the gamma gene on each
chromosome 11. The others are present in single copies. Therefore, each
cell has two beta globin genes, one on each of the two chromosomes 11 in
the cell. These two beta globin genes express their globin protein in a
quantity that precisely matches that of the four alpha globin genes. The
mechanism of this balanced expression is unknown.
Ontogeny of Hemoglobin Synthesis
Human Hemoglobins
| Embryonic hemoglobins |
Fetal hemoglobin |
Adult hemoglobins |
gower 1- zeta(2), epsilon(2)
gower 2- alpha(2), epsilon (2)
Portland- zeta(2), gamma (2) |
hemoglobin F- alpha(2), gamma(2) |
hemoglobin A- alpha(2), beta(2)
hemoglobin A2- alpha(2), delta(2) |
The globin genes are activated in sequence during development, moving from
5' to 3' on the chromosome. The zeta gene of the alpha globin gene cluster
is expressed only during the first few weeks of embryogensis. Thereafter,
the alpha globin genes take over. For the beta globin gene cluster, the
epsilon gene is expressed initially during embryogensis. The gamma gene
is expressed during fetal development. The combination of two alpha genes
and two gamma genes forms fetal hemoglobin, or hemoglobin F. Around the
time of birth, the production of gamma globin declines in concert with
a rise in beta globin synthesis. A significant amount of fetal hemoglobin
persists for seven or eight months after birth. Most people have only trace
amounts, if any, of fetal hemoglobin after infancy. The combination of
two alpha genes and two beta genes comprises the normal adult hemoglobin,
hemoglobin A. The delta gene, which is located between the gamma and beta
genes
on chromosome 11 produces a small amount of delta globin in children and
adults. The product of the delta globin gene is called hemoglobin A2, and
normally comprises less than 3% of hemoglobin in adults, is composed of
two alpha chains and two delta chains.
For more information, see "Hemoglobin: molecular, genetic, and clinical
aspects", Bunn and Forget, Saunders, 1986.








