revised April 27, 1998
An Overview of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia
(MSH)
[Charache, et al., 1995. The effect of hydroxyurea on the frequency
of painful crises in sickle cell anemia. N Engl J Med 332:1317-1322]
Study Design
The NHLBI organized the multicenter study of hydroxyurea in sickle
cell anemia to answer the question of whether hydroxyurea was clinically
useful to patients with sickle cell disease. Evaluating efficacy in sickle
cell disease is a difficult task due to the inherent variability of the
condition Further, some conditions genetically different from homozygous
HbS disease have a similar phenotype (e.g., sickle
ß-thalassemia and HbSC disease). In order to have a meaningful
study, the design committee established strict entry criteria:
Inclusion Criteria
-
Age 18 years or older.
-
Homozygous Hb SS disease. Of note, patients with homozygous HbSS disease
with coexisting alpha thalassemia were included in the study.
-
At least three painful crises reported to the physician in the year before
the study.
Exlusion Criteria
-
Pregnancy.
(hydroxyurea is teratogenic in rats.)
-
Patients with known narcotic addiction or who regularly consumed 30 or
more oxycodone tablets (or equivalent other narcotic) in two weeks.
-
Concurrent use of other potentially anti-sickling agents.
-
History of stroke in the preceding six years.
-
Prior treatment with hydroxyurea.
-
Antibody to the HIV virus.
-
Pre-existing depression of blood counts that would obscure bone marrow
suppression by the hydroxyurea.T
-
Transfusions resulting in a percent HbA greater than 15 on hemoglobin
electrophoresis at the start of the study.
(Patients on long-term chronic transfusion programs were ineligible for
the study.)
A total of 299 patients were enrolled in the study at 18 institutions
across the United States. Regular meetings of the participating investigators
were conducted before and during the study. The initial hydroxyurea dose
was 15 mg/kg/day, and was escalated in increments of 5 mg/kg every 12 weeks
to the maximum tolerated does (MTD: platelet or reticulocyte count that
falls to below 80,000/mm3.) If marrow depression
occurred, the hydroxyurea was held until the counts normalized. Hydroxyurea
was then was restarted at a dose less than that used previously by 2.5
mg/kg/day. One group of patients received placebo that was physically identical
to the hydroxyurea tablets. Drug pauses and adjustments were made in this
arm in order to maintain the double-blind nature of the investigation.
The primary outcome variable was pain. A painful crisis was defined
as a visit to a medical facility that lasted longer than four hours and
required parenterally administered narcotics for pain control. Other primary
outcome variables were acute chest syndrome,
priapism and hepatic sequestration crisis.
Results
Profile of the Patients in the MSH Study
|
Characteristic
|
Hydroxyurea
(N=152)
|
Placebo
(N=147)
|
| No. Crises in the year before before study entry (% patients) |
|
|
3-5
|
44
|
44
|
|
6-9
|
26
|
21
|
|
> or = 10
|
30
|
35
|
| Complications of sickle cell disease (% patients) |
|
|
Chest syndrome
|
66
|
65
|
|
Ankle ulcer
|
31
|
33
|
|
Aseptic necrosis of bone
|
20
|
22
|
|
Hemoglobin (g/dl)
|
8.4
|
8.5
|
|
White cells (10-3/mm3)
|
12.6
|
12.3
|
|
Platelets(10-3/mm3)
|
468
|
457
|
|
Reticulocytes (10-3/mm3)
|
327
|
325
|
|
Fetal hemoglobin (%)
|
5.0
|
5.2
|
|
F-cells (%)
|
33
|
33
|
|
Dense cells (%)
|
14.3
|
14.0
|
No significant difference existed between the two groups initially
with respect to sex, age, race or ethnic group, number of alpha globin
genes, or ß-globin haplotype. The blood counts were similar between
the two groups prior to treatment. After treatment was begun, one patient
in the hydroxyurea group was found to have sickle ß+-thalassemia
(a small amount of HbA was present).
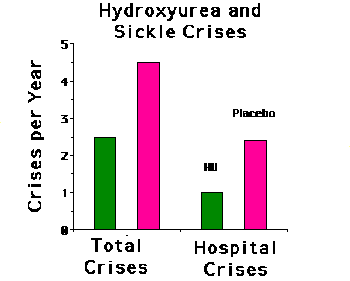 The median number of crises experienced by patients on hydroxyurea was
approximately half that of the control group. In addition, hydroxyurea
treatment reduced by more than half, the number of hospitalizations due
to sickle cell crises. Further supporting the efficacy of hydroxyurea in
preventing sickle cell crises, the median time to the first vaso-occlusive
crisis was 3.0 months in the hydroxyurea group compared to 1.5 months in
the placebo controls. The median time to the second crisis was likewise
greater in the patients on hydroxyurea relative to the controls (8.8 versus
4.6 months.) At the end of the study, 51% of the patients on hydroxyurea
were receiving maximal tolerated doses. The upper limit prescribed for
any patient was 35 mg/kg/day.
The median number of crises experienced by patients on hydroxyurea was
approximately half that of the control group. In addition, hydroxyurea
treatment reduced by more than half, the number of hospitalizations due
to sickle cell crises. Further supporting the efficacy of hydroxyurea in
preventing sickle cell crises, the median time to the first vaso-occlusive
crisis was 3.0 months in the hydroxyurea group compared to 1.5 months in
the placebo controls. The median time to the second crisis was likewise
greater in the patients on hydroxyurea relative to the controls (8.8 versus
4.6 months.) At the end of the study, 51% of the patients on hydroxyurea
were receiving maximal tolerated doses. The upper limit prescribed for
any patient was 35 mg/kg/day.
Acute chest syndrome is a leading cause of death of patients
with sickle cell disease. The patients on hydroxyurea had 25 episodes of
acute chest syndrome, while the placebo control patients experienced 51
episodes. The difference is significant with a P<0.001.
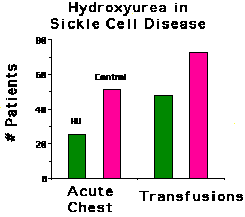 Patients in the study were transfused at the discretion of their physicians
(who were blinded to the treatment) as seemed medically appropriate. The
number of patients who required transfusion was less in the hydroxyurea
treated group compared to the controls. In addition, the patients on hydroxyurea
received 336 transfusions while the patients in the control group received
586 (P=0.004). This apparently paradoxical effect of hydroxyurea is likely
due diminished hemolysis in these patients relative to control. Some patients
on hydroxyurea had dramatic rises in their hemoglobin levels, in some cases
from the range of 7 gm/dl to as high as 13 gm/dl.
Patients in the study were transfused at the discretion of their physicians
(who were blinded to the treatment) as seemed medically appropriate. The
number of patients who required transfusion was less in the hydroxyurea
treated group compared to the controls. In addition, the patients on hydroxyurea
received 336 transfusions while the patients in the control group received
586 (P=0.004). This apparently paradoxical effect of hydroxyurea is likely
due diminished hemolysis in these patients relative to control. Some patients
on hydroxyurea had dramatic rises in their hemoglobin levels, in some cases
from the range of 7 gm/dl to as high as 13 gm/dl.
No death was related to treatment with hydroxyurea. Treatment
was permanently stopped in 14 patients in the hydroxyurea group (two of
whom showed myelotoxicity at a dose of 2.5 mg/kg/day). In the placebo group,
treatment was discontinued in six patients. Treatment was stopped temporarily
in nearly all the patients on hydroxyurea at some point because of myelosuppression.
Conclusion
This controlled trial made hydroxyurea the first drug of proven benefit
in the prevention of major problems caused by sickle cell disease. These
are:
-
Vaso-occlusive pain crisis
-
Acute chest syndrome
The study did not address the question of the ability of hydroxyurea
to reverse chronic organ damage (such as skin ulcers) nor prevent stroke.
Importantly, no significant side-effects of hydroxyurea therapy was noted.
Long-term effects are being monitored in the MSH follow-up study. Of greatest
concern in this regard is the possibility of neoplasic transformation by
a drug that alters DNA synthesis.
Go to Guidelines for Use of Hydroxyurea
Go to Overview of Sickle Cell Management
 The median number of crises experienced by patients on hydroxyurea was
approximately half that of the control group. In addition, hydroxyurea
treatment reduced by more than half, the number of hospitalizations due
to sickle cell crises. Further supporting the efficacy of hydroxyurea in
preventing sickle cell crises, the median time to the first vaso-occlusive
crisis was 3.0 months in the hydroxyurea group compared to 1.5 months in
the placebo controls. The median time to the second crisis was likewise
greater in the patients on hydroxyurea relative to the controls (8.8 versus
4.6 months.) At the end of the study, 51% of the patients on hydroxyurea
were receiving maximal tolerated doses. The upper limit prescribed for
any patient was 35 mg/kg/day.
The median number of crises experienced by patients on hydroxyurea was
approximately half that of the control group. In addition, hydroxyurea
treatment reduced by more than half, the number of hospitalizations due
to sickle cell crises. Further supporting the efficacy of hydroxyurea in
preventing sickle cell crises, the median time to the first vaso-occlusive
crisis was 3.0 months in the hydroxyurea group compared to 1.5 months in
the placebo controls. The median time to the second crisis was likewise
greater in the patients on hydroxyurea relative to the controls (8.8 versus
4.6 months.) At the end of the study, 51% of the patients on hydroxyurea
were receiving maximal tolerated doses. The upper limit prescribed for
any patient was 35 mg/kg/day.
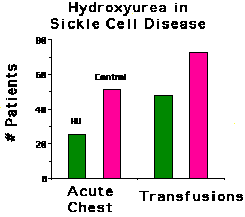 Patients in the study were transfused at the discretion of their physicians
(who were blinded to the treatment) as seemed medically appropriate. The
number of patients who required transfusion was less in the hydroxyurea
treated group compared to the controls. In addition, the patients on hydroxyurea
received 336 transfusions while the patients in the control group received
586 (P=0.004). This apparently paradoxical effect of hydroxyurea is likely
due diminished hemolysis in these patients relative to control. Some patients
on hydroxyurea had dramatic rises in their hemoglobin levels, in some cases
from the range of 7 gm/dl to as high as 13 gm/dl.
Patients in the study were transfused at the discretion of their physicians
(who were blinded to the treatment) as seemed medically appropriate. The
number of patients who required transfusion was less in the hydroxyurea
treated group compared to the controls. In addition, the patients on hydroxyurea
received 336 transfusions while the patients in the control group received
586 (P=0.004). This apparently paradoxical effect of hydroxyurea is likely
due diminished hemolysis in these patients relative to control. Some patients
on hydroxyurea had dramatic rises in their hemoglobin levels, in some cases
from the range of 7 gm/dl to as high as 13 gm/dl.