 >
>
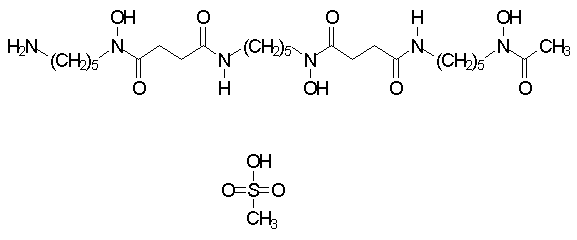
Many chelators are used in chemistry and industry. Only a few are clinically useful since most have dangerous side-effects. One important property required of clinically useful chelators is specificity. Since these drugs disperse diffusely in the body, they must bind the target metal ion preferentially over others. Desferrioxamine (Desferal®), for instance, can be used to treat iron overload since the drug binds iron with a large preference over other metal ions such as calcium (Kd=10-31 M for iron, Kd=10-9 M for calcium) (1).
One key clinical feature of iron chelators is the degree to which they are absorbed from the gastrointestinal tract. A clinically highly effective iron chelator such as desferrioxamine has the drawback of very poor absorption from the gastrointestinal tract (2). Consequently the drug must be given parenterally, as a continuous subcutaneous infusion, or as a continuous intravenous infusion (3, 4). The expensive medical paraphernalia required for desferrioxamine administration makes the treatment expensive, and curbs its availability in areas of the world where medical resources are limited. Even when the resources exist to support iron chelation with desferrioxamine, the intrusiveness of pumps and other paraphernalia often impedes patient compliance (5). For these reasons, an intensive search for orally active iron chelators is being conducted by a number of medical researchers.
Because of its ability to participate in chemical reactions that involve the shift of electrons between molecules (reduction-oxidation or redox reactions), the body tightly regulates iron. When iron is tightly bound to a chelator molecule, be it a protein or a small chemical, the reactivity of the iron is greatly dampened. The key iron storage protein in the body is ferritin. Ferritin is a very large spherical molecule (6). Iron is deposited as semi-crystalline deposits inside these protein "vaults". Iron that is sequestered within ferritin is metabolically inactive.
The iron deposits in patients who have received multiple blood transfusions for chronic anemia, such as thalassemia, can exceed the storage and detoxification capacity of ferritin. Consequently, "free" (or more accurately, loosely bound) iron begins to accumulate in tissues and blood. This "free" iron can catalyze the formation of very injurious compounds, such as the hydroxyl radical (.OH) from compounds such as hydrogen peroxide, which are normal metabolic byproducts (Fenton reaction) (7).
The hydroxyl radical is highly reactive, and attacks lipids, proteins and DNA (8). The initial reaction with each of these molecules is the formation of peroxides (e.g., lipid peroxides) that can interact with other molecules to form cross links. These cross-linked molecules perform their normal functions either poorly or not at all.
Lipids
Peroxidaiton promotes cross links in membrane lipids, creating islands or domains
of dysfunctional molecules. Cell membranes, which consist primarily of
lipids, stiffen and acquire odd shapes. This is particularly problematic
for red cells, which have no nucleus. Unlike most other cells, red cells
cannot repair membrane damage. The red cells of patients with thalassemia
or sickle cell disease loose the elasticity needed to pass through the
microcirculation (9). For patients with sickle cell disease, this
exacerbates the problem of microvascular vaso-occlusion. These damaged
red cells are removed by reticuloendothelial cells, most prominently in
the spleen.
Proteins
Protein cross linking can create protein clusters, particularly in
membranes (10,11). Again, red cells are particularly susceptible
to such damage, lacking membrane repair mechanisms. The cells of the immune
system recognize these protein clusters as being abnormal. Antibodies to
these clusters (termed "membrane senescence antibodies") promote removal
of damaged red cells from the circulation. The result is enhanced hemolysis. Oxidation of band 3, the red cell anion transport channel, disturbances the osmotic balance of red cells and impairs their function.
DNA
DNA cross-links can impair cell replication, leading to cell death.
The degree of cross-linking produced by reactive oxygen species in patients
with iron overload generally is relatively small and probably relatively unimportant.
Although red cells are very susceptible to iron-mediated cell injury, they do not bear the assault of reactive oxygen species alone. Damage to cells in other organs accumulates gradually, and eventually becomes clinically significant. Hepatocytes, the primary component cells of the liver, are the major storage site for body iron. With iron overload, these cells are relentlessly bombarded by reactive oxygen species and eventually die (12). They are replaced by fibroblast cells. The collagen laid down by fibroblasts produces liver fibrosis and, eventually, cirrhosis.
Likewise, cardiac cells are damaged with iron overload (13). Normal cardiac function requires the coordinate activity of all the cells in the heart. Damaged, poorly-functioning cells often fail in this regard. The clinical manifestations include congestive heart failure (due to injury to myocytes) and arrythmias (due to damage to the cells of the cardiac conducting system) (14, 15). Either can be deadly.
Patients with some disorders develop iron overload due to repeated transfusions, afterwhich the underlying disorder is corrected. For instance, some people with aplastic anemia who receive a bone marrow transplant require many transfusions for support until the graft matures. Thereafter, they have a normal hematocrit. Chelators can remove all the excess iron in this setting.
Most patients with transfusional iron overload require transfusions indefinately. Examples of this include people with thalassemia major and some froms of myelodysplasia. Since each unit of blood deposits about 230 mg of iron, most patients who require, for instance, 2 units of blood per month will have at most a very slightly negative iron balance with chelation therapy. The most widely used iron chelator, desferrioxamine, removes somewhere between 30 and 70 mg of iron per day. Protection of patients with ongoing transfusion requirements solely by removal of excess iron is uncommon.
Since neutralization of free iron is essential to protect cells, a molecule such as desferrioxamine has the advantage of inactivating iron as part of a 1:1 molecular complex. On the other hand, bidentate chelators (C) can produce partial reaction products with iron (Fe):
With a bidentate iron chelator, a spectrum of chemical species will exist, of which a minority is inactive. A large chemical excess of chelator is needed to push the reaction toward completion, the formation of the FeC3 (inactive) product.
 > > |
Intensive exploration for an orally active iron chelator is currently
under way. The only drug actively considered at present is deferiprone,
or "L1". Investigators in Canada, the US, Italy, Greece, and India have
studied this agent (19, 20, 21). Presently, no consensus exists
on the utility of L1. The drug does not mobilize iron as efficiently as
desferrioxamine. This shortcoming could, however, be balanced by better
compliance. Some serious side-effects, such as agranulocytosis, occur with
L1 (22). The degree to which these will limit its utility is presently
unclear. Finally, in contrast to the hexidentate chelating capacity of
desferrioxamine, L1 is a bidentate chelator. Intermediate chelation products
could continue to produce cell and organ injury in patients treated with
this drug. The issue of progression of liver damage in patients who use deferiprone is at the center of the current controversy over the drug (23). Further investigation is warranted to determine where L1 fits
in the clinical armamentarium.